| Catalog No. | HB996016 |
|---|
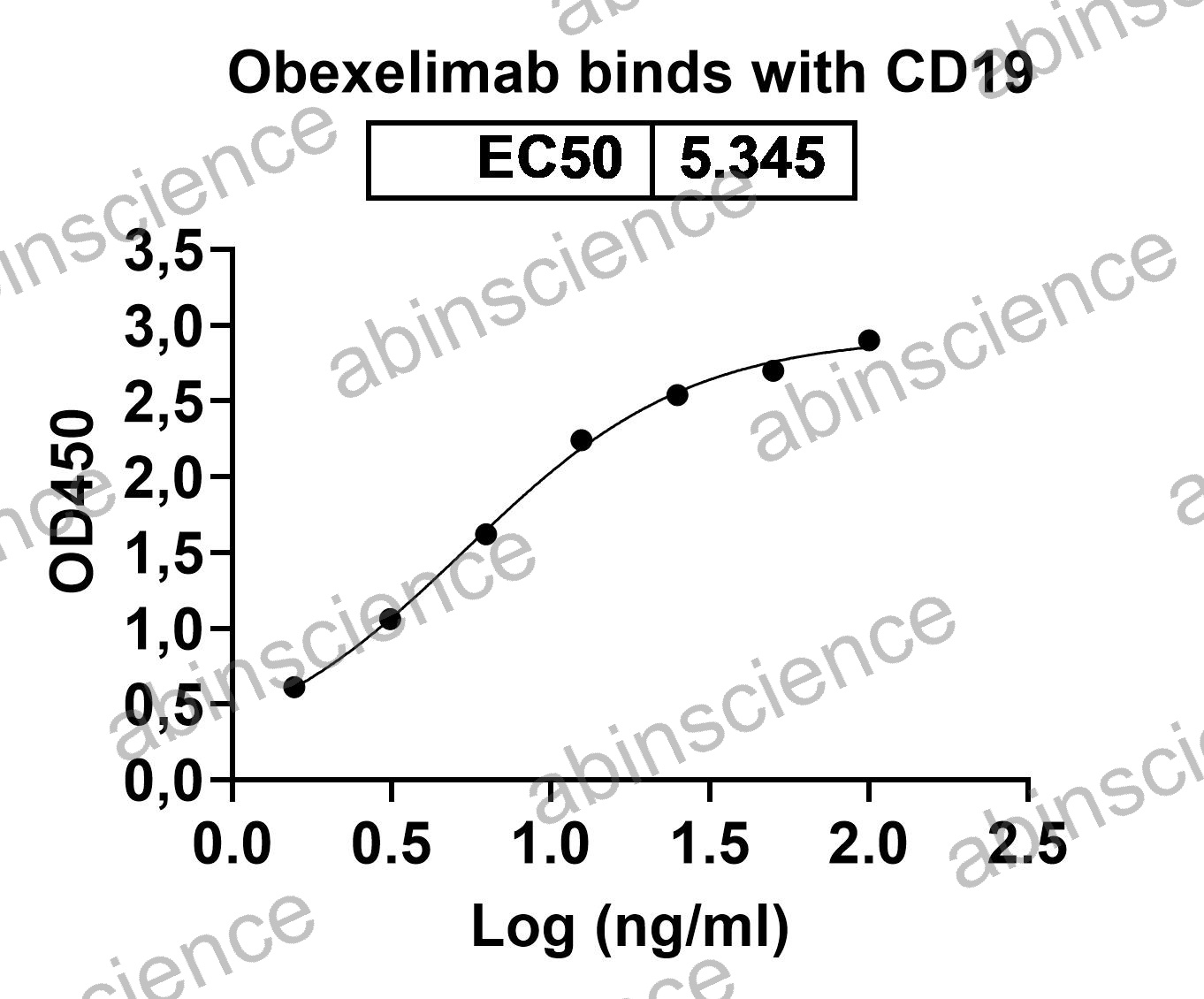
| Description | Coltuximab ravtansine (SAR3419) is an antibody-drug conjugate (ADC) targeting CD19 created by conjugating a derivative of the potent microtubule-acting cytotoxic agent, maytansine, to a version of the anti-CD19 antibody, anti-B4, that was humanized as an IgG1 by variable domain resurfacing. Four different linker-maytansinoid constructs were synthesized (average ∼3.5 maytansinoids/antibody for each) to evaluate the impact of linker-payload design on the activity of the maytansinoid-ADCs targeting CD19. Anti-CD19 humanized monoclonal antibody conjugated to DM4. |
|---|
| Species reactivity | Human |
|---|
| Applications | ELISA, Bioactivity: FACS, Functional assay, Research in vivo |
|---|
| Host species | Chimeric |
|---|
| Isotype | IgG1-kappa |
|---|
| Expression system | Mammalian Cells |
|---|
| Species | Human |
|---|
| Clonality | Monoclonal |
|---|
| Target | CD19, Differentiation antigen CD19, T-cell surface antigen Leu-12, B-lymphocyte antigen CD19, B-lymphocyte surface antigen B4 |
|---|
| Endotoxin level | Please contact with the lab for this information. |
|---|
| Purity | >95% as determined by SDS-PAGE. |
|---|
| Purification | Protein A/G purified from cell culture supernatant. |
|---|
| Accession | P15391 |
|---|
| Form | Liquid |
|---|
| Storage buffer | 0.01M PBS, pH 7.4. |
|---|
| Stability and Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term. |
|---|
| Alternate Names | SAR3419, 1269764-99-9 |
|---|
| Background | Budoprutug is an anti-CD19 human IgG1 κ monoclonal antibody. Recommend Isotype Controls: Human IgG1 kappa, Isotype Control (HY-P99001).
• An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer., PMID:30680780
• CAR-T cell therapy., PMID:32586666
• A nondepleting anti-CD19 antibody impairs B cell function and inhibits autoimmune diseases., PMID:37427592
• B-cell depletion in vitro and in vivo with an afucosylated anti-CD19 antibody., PMID:20605905
• Efficacy and safety of CD19-directed CAR-T cell therapies in patients with relapsed/refractory aggressive B-cell lymphomas: Observations from the JULIET, ZUMA-1, and TRANSCEND trials., PMID:34310745
• CD19-CAR trials., PMID:24667955
• Optimized CD19/CD22/CD3 antibody., PMID:36264591
• [Not Available]., PMID:30686355
• Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma., PMID:33035333
• Reducing Ex Vivo Culture Improves the Antileukemic Activity of Chimeric Antigen Receptor (CAR) T Cells., PMID:30030295
|
|---|
| Note | For research use only. Not suitable for clinical or therapeutic use. |
|---|