| Catalog No. | VK421036 |
| Host species | Humanized |
| Species reactivity | HRSV-A |
| Form | Liquid |
| Storage buffer | 0.01M PBS, pH 7.4. |
| Purity | >95% purity as determined by SDS-PAGE. |
| Clonality | Monoclonal |
| Isotype | IgG1, kappa |
| Applications | ELISA, Bioactivity: FACS, Functional assay, Research in vivo |
| Target | F, Fusion glycoprotein F0, Fusion glycoprotein F2, p27, Intervening segment, Pep27, Peptide 27, Fusion glycoprotein F1 |
| Purification | Protein A/G purified from cell culture supernatant. |
| Endotoxin level | Please contact the lab for this information. |
| Expression system | Mammalian Cells |
| Accession | P03420 |
| Stability and Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C for short-term storage (1-2 weeks). Store at -20°C for up to 12 months. For long-term storage, store at -80°C. |
| Alternative name | MEDI-524, NUMAX, NISTmAb, CR9503, 677010-34-3 |
| Note | For research use only. Not suitable for clinical or therapeutic use. |
Research Grade Motavizumab
Overview
Description
Motavizumab (MEDI-524, NUMAX, NISTmAb) is a second generation monoclonal antibody (mAb) derived from palivizumab (Synagis) using affinity maturation techniques. Motavizumab is currently undergoing US Food and Drug Administration review as a treatment for respiratory syncytial virus (RSV) prophylaxis. It has been evaluated in large-scale clinical studies, and has demonstrated efficacy in reducing the disease burden of RSV in high-risk infant populations. Motavizumab (MEDI-524, NUMAX, NISTmAb) has higher affinity and a longer half-life, was effective in reducing RSV hospitalization in high-risk full-term infants in the US, but was not licensed due to safety concerns (allergic reactions).
Images
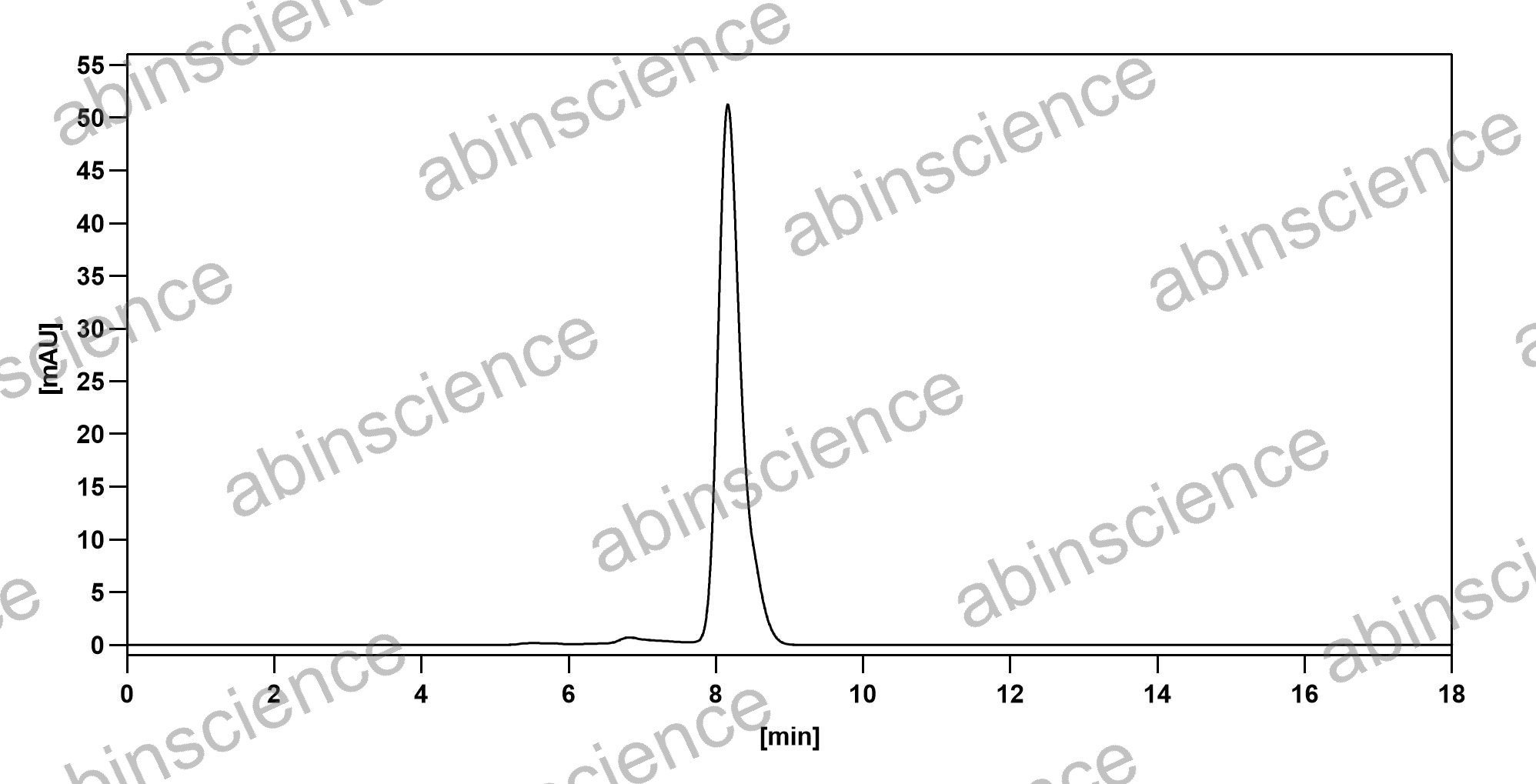 SEC-HPLC |
SEC-HPLC detection for Research Grade Motavizumab. |
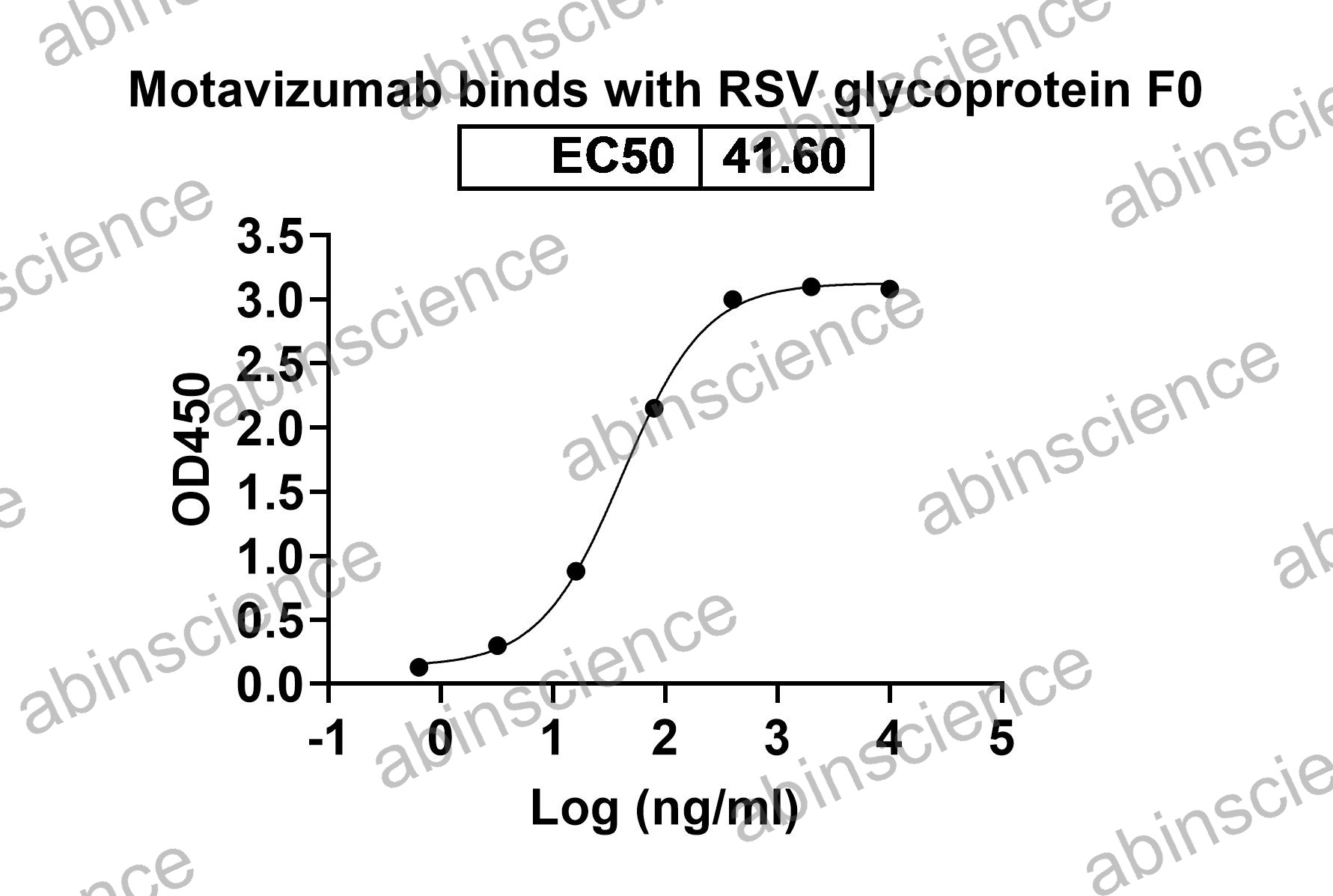 Bioactivity |
Detects F/Fusion glycoprotein F0 in indirect ELISAs. |
 SDS-PAGE |
SDS-PAGE for Research Grade Motavizumab. |