On April 10, 2025, California health officials confirmed multiple fatalities linked to hantavirus infection, drawing renewed attention to Hemorrhagic Fever with Renal Syndrome (HFRS)—a zoonotic disease with case fatality rates up to 15%, primarily caused by Hantaan virus (HTNV) in Asia. Though largely overlooked in the West, HTNV remains endemic in China, Korea, and Russia, with seasonal outbreaks driven by rodent population dynamics and human exposure. Recent data from China indicate approximately 10,000–15,000 HFRS cases annually, predominantly in northeastern provinces like Heilongjiang and Jilin, linked to rural agricultural activities and climatic factors like heavy rainfall, which amplify rodent-human contact.
From a virological perspective, Hantaan virus represents not only a persistent public health threat but also a complex molecular system whose structural and functional biology offers opportunities for diagnostic development, immunotherapy, and viral pathogenesis studies.
Virological Classification and Structure of Hantaan Virus
Hantaan virus is a prototypic member of the Orthohantavirus genus, within the Hantaviridae family (Bunyavirales order). It is a tri-segmented, enveloped, negative-sense RNA virus, with each genomic segment encoding a critical protein for its life cycle and pathogenesis:
| Segment |
Encoded Protein |
Molecular Function |
| S (Small) |
Nucleoprotein (N) |
Encapsidation of viral RNA; immune recognition; modulates host immune signaling |
| M (Medium) |
Glycoproteins Gn & Gc |
Mediates host cell attachment, endocytosis, and fusion; key targets for neutralizing antibodies |
| L (Large) |
RNA-dependent RNA polymerase (RdRp) |
Catalyzes transcription and replication of viral RNA; lacks proofreading, allowing adaptation |
The viral envelope is studded with heterodimeric Gn-Gc spikes, which bind to β3-integrins and protocadherin receptors on human endothelial cells, a key determinant of the virus's endothelial tropism and vascular leak pathology. Viral particles range from 75–210 nm, with a mean diameter of ~120 nm, and display pleomorphic morphology under electron microscopy.
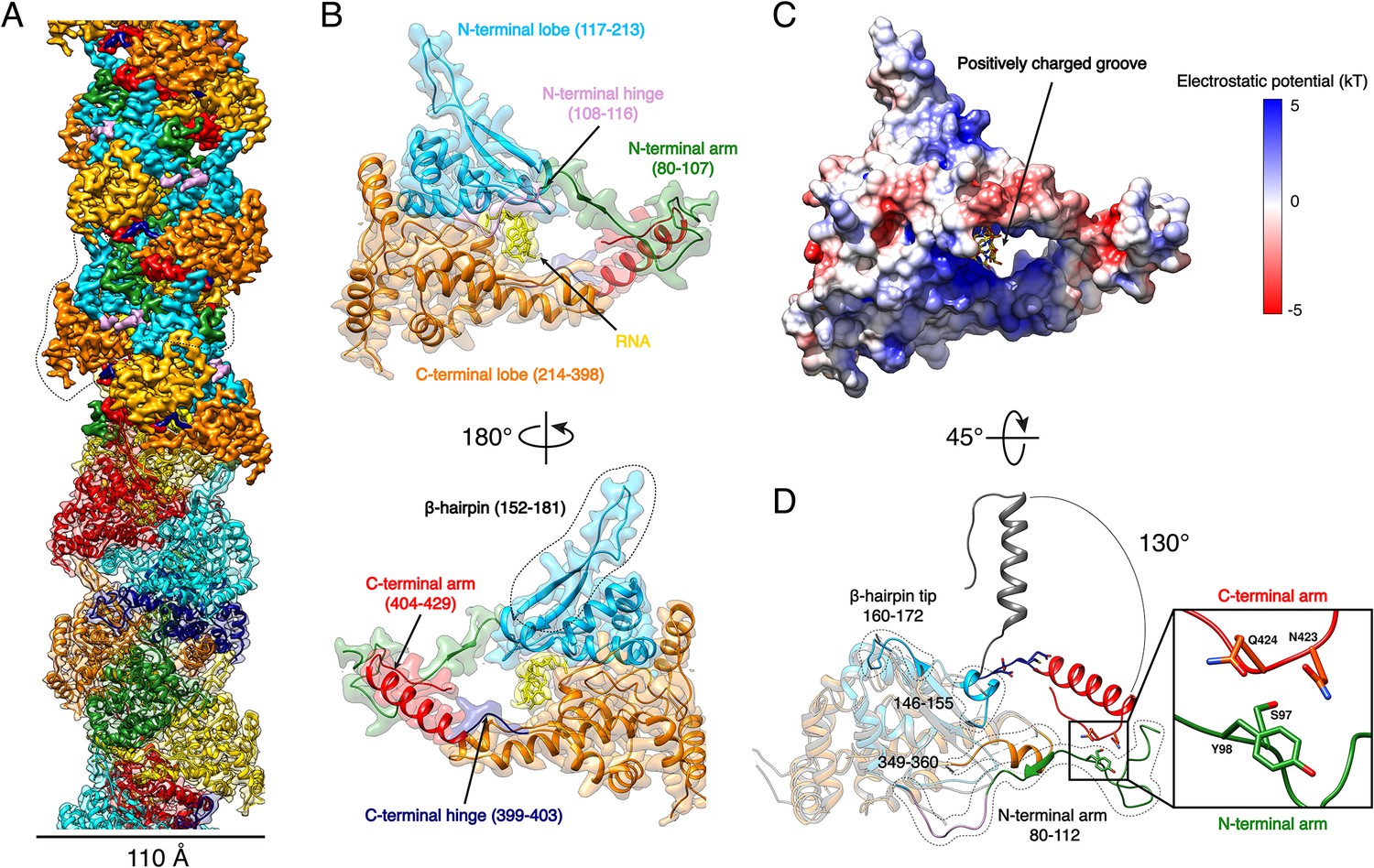
Figure 1: Cryo-EM structure of Hantaan virus, highlighting the helical assembly of nucleoprotein-RNA complexes within the envelope, alongside Gn-Gc glycoproteins. Source: Arragain et al., 2019
Mechanism of Cellular Entry and Replication
Following inhalation of virus-laden aerosols from infected rodent excreta, HTNV primarily targets endothelial cells via receptor-mediated endocytosis. The low pH of late endosomes induces conformational changes in Gc, enabling class II fusion of viral and host membranes. The nucleocapsid is released into the cytoplasm, where RdRp initiates cap-snatching-dependent transcription and replication.
Unlike many acute viruses, HTNV exhibits a non-cytolytic replication strategy, contributing to its prolonged incubation (1–2 weeks) and the biphasic clinical course of HFRS—initial febrile illness followed by renal impairment, proteinuria, and, in severe cases, hemorrhage and shock.
Immune Evasion Strategies of Hantaan Virus
HTNV has evolved sophisticated mechanisms to evade host immunity, contributing to its persistence and severity. The nucleoprotein (N) inhibits interferon-beta (IFN-β) production by sequestering importin-α, disrupting NF-κB signaling, a mechanism supported by cryo-EM studies revealing N-importin-α binding interfaces. Additionally, the non-structural protein encoded by alternative reading frames of the S segment antagonizes RIG-I-like receptor pathways, dampening innate immune responses and reducing CXCL10-mediated T-cell recruitment. These strategies delay adaptive immunity, particularly T-cell activation, allowing prolonged viral replication in endothelial cells and exacerbating vascular leakage.
Research Bottlenecks and the Need for Validated Tools
Despite its high morbidity and mortality in endemic regions, HTNV remains understudied due to biosafety constraints (typically BSL-3), lack of standardized animal models, and absence of licensed antivirals. Moreover, its slow in vitro replication kinetics and broad serological cross-reactivity across hantaviruses complicate diagnostic assay development. Emerging technologies, such as CRISPR-Cas-based detection systems, show promise for rapid HTNV diagnostics, offering higher specificity than traditional serology.
Validated, recombinant viral proteins are critical for overcoming these hurdles, enabling:
- ■Elucidating virus-host interactions at the molecular level
- ■Mapping antigenic epitopes for vaccine and neutralization studies
- ■Developing serodiagnostics (ELISA, LFIAs, Western blot)
- ■Screening antiviral compounds in high-throughput systems
Therapeutic and Vaccine Development for Hantaan Virus
Currently, no licensed antivirals or vaccines are available for HTNV. Ribavirin has shown limited efficacy in early-stage HFRS but is hindered by toxicity and narrow therapeutic windows. Promising candidates include nucleoside analogs targeting RdRp and monoclonal antibodies against Gn/Gc, though resistance remains a concern for long-term use. Vaccine efforts focus on recombinant Gn/Gc-based platforms, with virus-like particles (VLPs) and DNA vaccines showing immunogenicity in preclinical models. A phase I trial for a DNA vaccine encoding HTNV glycoproteins reported detectable neutralizing antibodies in 60% of participants, though challenges remain in eliciting durable immunity across diverse populations.
abinScience’s HTNV Protein Portfolio: Precision Tools for Molecular Virology
High-quality recombinant proteins and antibodies are indispensable for advancing HTNV research. abinScience provides a curated panel of recombinant Hantaan virus proteins and antibodies, expressed in eukaryotic systems to ensure proper folding and glycosylation for functional and immunological relevance. These tools support studies on host-receptor interactions, neutralization mapping, and serological diagnostics. Our catalog includes:
| Type |
Catalog No. |
Product Name |
| Protein |
VK6460122 |
Recombinant BCCV Gc/Glycoprotein C Protein |
| VK6460222 |
Recombinant BCCV Gn/Glycoprotein N Protein |
| VK484012 |
Recombinant BCCV N/Nucleoprotein Protein, N-His |
| Antibody |
VK6460201 |
Anti-BCCV Gc/Glycoprotein C Polyclonal Antibody |
| VK4840104 |
Anti-BCCV Gn/Glycoprotein N Polyclonal Antibody |
| VK4840144 |
Anti-BCCV N/Nucleoprotein Polyclonal Antibody |
| VK6460100 |
InVivoMAb Anti-Orthohantavirus puumalaensis/Hantaan virus Glycoprotein |
| VK6460300 |
InVivoMAb Anti-Hantaan virus Glycoprotein |
| VK6460143 |
Anti-Hantaan virus/HTNV GP Antibody (3C11) |
| VK6460223 |
Anti-Hantaan virus/HTNV GP Antibody (A5) |
| VK6460106 |
Research Grade Anti-Orthohantavirus puumalaensis |
| VK6460036 |
Research Grade Anti-Orthohantavirus puumalaensis |
All products are QC-verified via SDS-PAGE, Western blot, and ELISA, with custom conjugation and bulk supply options available.
Full catalog at: abinScience Hantaan Virus Tools
The Dual Role of HTNV: Threat and Scientific Window
While HTNV continues to pose a zoonotic threat, its replication strategy, immune evasion, and vascular pathology offer valuable insights for broader virological study—including non-cytopathic virus persistence, RNA polymerase dynamics, and host-viral coadaptation.
Leveraging recombinant tools to dissect HTNV biology may also inform strategies against other segmented negative-strand RNA viruses, including Lassa virus and arenaviruses.
Empowering Molecular Discovery in Hantavirus Research
abinScience is committed to supporting the scientific community in high-containment virology, providing precision tools to accelerate HTNV discovery and countermeasure development. Whether you're focused on structural virology, immunogen design, or pathogenesis modeling, our reagents deliver quality and consistency.
Contact us for datasheets, quotations, or collaboration opportunities at support@abinscience.com.
Hantavirus may be resurging—but with the right tools, science is one step ahead.
References
[1]Arragain, B., Effantin, G., Gerlach, P., Vergnes, A., Zuber, G., Weissenhorn, W., ... & Fender, P. (2019). Cryo-EM structure of the Hantaan virus nucleoprotein-RNA complex reveals the mechanism of RNA encapsidation. eLife, 8, e43075. https://doi.org/10.7554/eLife.43075
[2]Guardado-Calvo, P., Bignon, E. A., Stettner, E., Jeffers, S. A., Pérez-Vargas, J., Pehau-Arnaudet, G., ... & Rey, F. A. (2016). Crystal structure of the Hantaan virus Gc fusion protein in its prefusion conformation. Nature, 534(7606), 260–263. https://doi.org/10.1038/nature18615
[3]Li, S., Rissanen, I., Zeltina, A., Hepojoki, J., Raghwani, J., Harlos, K., ... & Bowden, T. A. (2016). A molecular-level account of the antigenic drift of Hantaan virus glycoproteins. Journal of Virology, 90(9), 4666–4677. https://doi.org/10.1128/JVI.00136-16





